

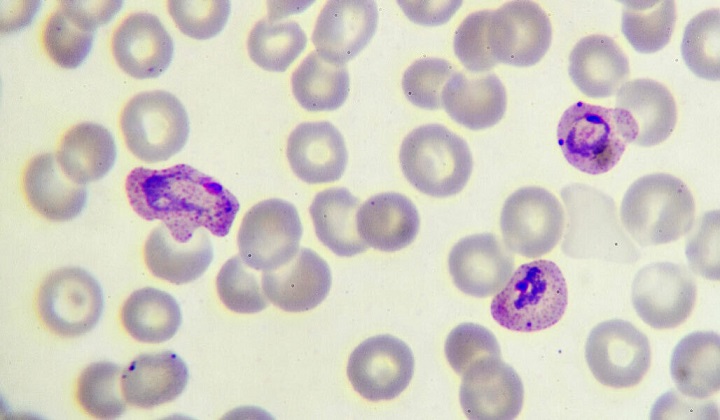
Once transmitted to a person via mosquito bite, the malaria-causing parasite, Plasmodium falciparum (shown purple), multiplies within red blood cells.
JARUN011/ISTOCK/GETTY IMAGES PLUS
The parasite may turn genes on or off to allow the spleen to clean up infected blood cells.
Malaria parasites survive tough times by not being too clingy.
During Africa’s dry season, when mosquitoes are scarce, malaria parasites have a hard time spreading to new hosts. So the parasites hide out in the human body by keeping the cells they infect from clinging to blood vessels, researchers report October 26 in Nature Medicine. This way, infected cells get removed from circulation and parasite levels in the body remain low, making people less sick and allowing the parasite to persist undetected.
Doctors have long observed that symptoms of malaria, a deadly mosquito-borne infection, tend to wane during the dry season, which runs from January to May. But the reason has been unclear.
Keeping a low profile during dry months is a successful strategy for the parasite, says Martin Rono, a parasitologist at the KEMRI-Wellcome Trust in Kilifi, Kenya, who was not involved in the work. Knowing how malaria parasites persist without causing disease, until mosquitoes return to ferry the organisms from an infected person to the next victim could help efforts to control malaria during the dry season.
Plasmodium falciparum, the parasite responsible for malaria, infects red blood cells as part of a complex life cycle. Once inside a cell, the parasite produces proteins that dock on the cell’s exterior and make it stick to blood vessels so that it won’t be carried to the spleen, where it would otherwise get removed from the body.
Typically, only the early life stages of the parasite circulate in the blood, while older parasites thrive inside red blood cells adhered to blood vessels, says Silvia Portugal, a biologist who led the work while at Heidelberg University Hospital in Germany. “That’s textbook, so it was very surprising” to see that dry-season parasites behaved differently in the lab, she says—the cells weren’t sticking.
Portugal, now at the Max Planck Institute for Infection Biology in Berlin, and her colleagues identified around 600 people in Mali infected with malaria in 2017 and 2018. First, the team ruled out two other ideas to explain the parasite’s seasonality: genetic diversity and immunity. Genetically distinct parasites did not hit at different seasons, nor did the host immune system suppress the parasite’s growth, the team found.
But when the researchers compared which genes were turned on or off in samples taken from asymptomatic people in the dry season and symptomatic people in the wet season, they saw that 1,607 genes had distinct seasonal patterns. In the dry season, 1,131 genes were turned on that were off in wet-season parasites. Another 476 were turned off in dry-season parasites, suggesting that when the wet season ends, P. falciparum may alter its genetics to make red blood cells less sticky. That allows the parasite to replicate and persist without setting off alarm bells that alert the immune system to fight the infection.
Blood cells infected with malaria use certain proteins to adhere to blood vessels almost like Velcro, Portugal says. The loss of stickiness could be because the parasite makes fewer of these proteins, or because the proteins are different in some way.
It’s still unclear, however, which specific genes are involved in the shift. One issue is that the researchers compared parasites from two different life stages, says Abdirahman Abdi, a parasitologist also at the KEMRI-Wellcome Trust. To narrow down which genes may be affecting stickiness, he says, the researchers may need to compare genetic activity in parasites at the same stage.
CITATIONS:
C. M. Andrade et al. Increased circulation time of Plasmodium falciparum underlies persistent asymptomatic infection in the dry season. Nature Medicine. Published online October 26, 2020. doi: 10.1038/s41591-020-1084-0.
Source:
https://www.sciencenews.org/article/malaria-parasite-mosquitoes-genetics-immune-system

Scheduled Server Maintenance and System Downtime Notice Dec 16, 2025
Celebrating CM Editorial Board Members Recognized in the Wor... Oct 10, 2025

Food Science and Engineering Now Indexed in CAS Database Aug 20, 2025
Contemporary Mathematics Achieves Significant Milestone in 2... Jun 19, 2025

Three Journals under Universal Wiser Publisher are Newly Ind... Apr 21, 2025